Pacific Northwest National Laboratory
Aug 11, 2011
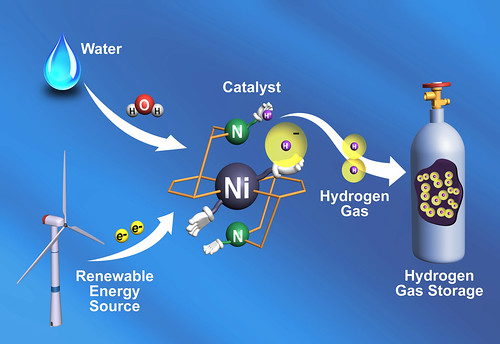
Looking to nature for their muse, researchers have used a common protein to guide the design of a material that can make energy-storing hydrogen gas. The synthetic material works 10 times faster than the original protein found in water-dwelling microbes, the researchers report in the August 12 issue of the journal Science, clocking in at 100,000 molecules of hydrogen gas every second.
This step is just one part of a series of reactions to split water and make hydrogen gas, but the researchers say the result shows they can learn from nature how to control those reactions to make durable synthetic catalysts for energy storage, such as in fuel cells.
In addition, the natural protein, an enzyme, uses inexpensive, abundant metals in its design, which the team copied. Currently, these materials — called catalysts, because they spur reactions along — rely on expensive metals such as platinum.
"This nickel-based catalyst is really very fast," said coauthor Morris Bullock of the Department of Energy's Pacific Northwest National Laboratory. "It's about a hundred times faster than the previous catalyst record holder. And from nature, we knew it could be done with abundant and inexpensive nickel or iron."
To read more click here...
Friday, August 12, 2011
Catalyst that makes hydrogen gas breaks speed record
Labels:
Education,
Energy,
Materials,
Research and Development,
Technology
Subscribe to:
Post Comments (Atom)







No comments:
Post a Comment